AbbVie announced today that U-EXCEED achieved positive top-line results. The U-EXCEED study is divided into three parts. The first phase is a 12-week placebo-controlled induction period. Part 2 of U-EXCEED is an open-label arm that evaluated an additional group of patients who received upadacitinib 45 mg for 12 weeks. At week 12, upadacitinib (45 mg once daily) achieved both primary endpoints of clinical remission and endoscopic response in a Phase 3 induction study.
Patients with moderate-to-severe Crohn’s disease who had an inadequate response or were intolerant to biologic therapy were enrolled in the U-EXCEED study, with more than 60% having previously failed two or more biologics. The U-EXCEED study is the first of two Phase 3 induction trials that will assess the safety and efficacy of upadacitinib in adults with moderate to severe Crohn’s disease.
Clinical remission was assessed in U-EXCEED using the Crohn’s Disease Activity Index (CDAI) and patient-reported symptoms of stool frequency/abdominal pain (SF/AP). When compared to placebo, a significantly higher proportion of patients treated with a 12-week induction regimen of upadacitinib 45 mg daily achieved clinical remission per CDAI at week 12 (39 percent versus 21 percent; p 0.0001). Similar outcomes were observed for clinical remission per SF/AP (40 percent in patients receiving upadacitinib versus 14 percent in patients receiving placebo; p0.0001). In this study, endoscopy was used to assess each patient for improvement in the intestinal mucosa.

When compared to the placebo group, a significantly higher percentage of patients receiving upadacitinib 45 mg at week 12 had an endoscopic response (35 percent versus 4 percent; p 0.0001).
When compared to placebo at week 12.1 among patients taking corticosteroids at baseline, a significantly higher percentage of patients receiving upadacitinib 45 mg attained steroid-free clinical remission per CDAI and per SF/AP.
Both early symptom improvement as measured by CR-100 (defined as a reduction of CDAI 100 points from baseline) at week 2 and clinical remission at week 4 were significantly more common in patients receiving upadacitinib than in those receiving placebo.
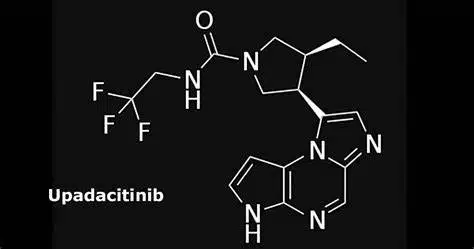
Upadacitinib
AbbVie Inc. is an American pharmaceutical company established in 2013. Known previously as an Abbott Laboratories spin-off, today they are one of the biggest pharmaceutical companies in the world, frequently placing in the top five by revenue.

RINVOQ, a selective and reversible JAK inhibitor that was discovered and created by AbbVie researchers, is being investigated in some immune-mediated inflammatory Enzymatic and cellular assays revealed that RINVOQ had a stronger inhibitory effect on JAK-1 than on JAK-2, JAK-3, and TYK-2The importance of inhibiting particular JAK enzymes for therapeutic efficacy is not currently understood.
The U.S. Food and Drug Administration (FDA) has approved RINVOQ 15 mg for use in treating adults with moderately to severely active rheumatoid arthritis who have not responded well to or are intolerant to one or more TNF blockers.
The U.S. Food and Drug Administration (FDA) has approved RINVOQ 15 mg for use in treating adults with moderately to severely active rheumatoid arthritis who have not responded well to or are intolerant to one or more TNF blockers.
The European Commission has also approved RINVOQ for use in moderate-to-severe atopic dermatitis in adults (15 mg and 30 mg) and adolescents (15 mg).

Adults with moderate to severely active rheumatoid arthritis, adult patients with active psoriatic arthritis, and adult patients with active ankylosing spondylitis are all eligible to use RINVOQ 15 mg, according to the European Commission.
RINVOQ is currently undergoing phase 3 trials for rheumatoid arthritis, giant cell arteritis, atopic dermatitis, psoriatic arthritis, axial spondyloarthritis, Crohn’s disease, ulcerative colitis, and Takayasu.
An immune-mediated chronic inflammatory bowel illness called ulcerative colitis can put a great deal of strain on patients and frequently leave them disabled. The CHMP’s favorable view is supported by findings from three Phase 3 studies, two of which were conducted for induction and one for maintenance.