The Centre makes it mandatory to test Indian export samples of cough syrup following quality concerns in abroad.

New Delhi: Starting from June 1, cough syrup exporters will be required to have their products tested at designated government laboratories before they can obtain permission for exporting them. This directive was issued in response to quality concerns of cough syrups exported by Indian companies that were raised internationally.
Table of Contents
According to a notification from the Directorate General of Foreign Trade (DGFT) on Tuesday, cough syrup exports will only be allowed if export samples are tested and accompanied by a ‘Certificate of Analysis’ issued by seven specific central government labs.
These labs include the Indian Pharmacopoeia Commission (Ghaziabad), Regional Drug Testing Lab (RDTL – Chandigarh), RDTL (Guwahati), Central Drugs Lab (CDL – Kolkata), National Accreditation Board for Testing and Calibration Laboratories (NABL) and Central Drug Testing Laboratory (CDTL – Mumbai, Chennai, Hyderabad) accredited drug testing labs of state governments.
An official explained that this pre-quality check requirement for exported cough syrup formulations aims to emphasize India’s commitment to ensuring the quality of pharmaceutical products.
Contaminants present in alleged syrups

Indian tests conducted on cough syrups manufactured by Maiden Pharmaceuticals Ltd in India, which were associated with the deaths of children in Gambia did not reveal any presence of toxins. However, contaminants were found in several drugs, which were linked to deaths in Uzbekistan, produced by Marion Biotech.
The finished cough syrup products will undergo testing at laboratories before they are permitted for export. The Ministry of Health and Family Welfare (MoHFW) will collaborate with state governments and exporters to facilitate the smooth implementation of this notification.
The decision to introduce pre-testing of cough syrup exports follows incidents such as the recall of eye drops by Global Pharma Healthcare in Tamil Nadu in February and reports linking Indian-made cough syrups to the deaths of 19 in Uzbekistan 70 children in Gambia in the previous year.
Additionally, the Health Minister, along with federal and state regulators, held a brainstorming session earlier this year in Hyderabad, in an effort to find a solution to the issue of exported cough syrups causing fatalities among children. This information was outlined in a document from the prime minister’s office dated May 15.
India’s position in Pharma production
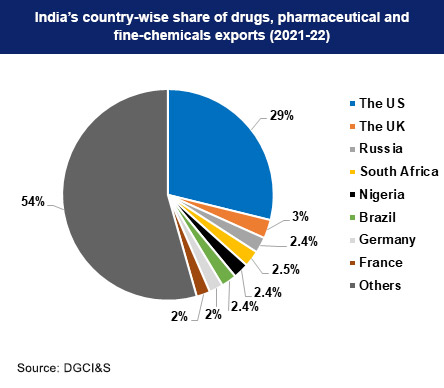
Image source: IBEF
The Indian pharmaceutical industry holds a prominent position as a manufacturer and exporter of medical products worldwide, supplying both developed countries and Low and Middle Income Countries (LMICs). India is the largest global provider of generic drugs, meeting over 50% of the global demand for vaccines and around 40% of the generic demand in the US. Additionally, India supplies about 25% of all medicine in the UK.
India exported cough syrups worth $17.6 billion in the financial year 2022-23 from an increase from $17 billion in 2021-22.
In terms of pharmaceutical production, India ranks third globally by volume and 14th by value. The industry comprises 3,000 drug companies and approximately 10,500 manufacturing units. It plays a crucial role in ensuring the availability and affordability of high-quality medicines worldwide. Indian pharmaceutical firms currently supply over 80% of the antiretroviral drugs used globally to combat AIDS.













