India accounts for the majority of cervical cancer fatalities worldwide with 80,000 new cases per year. How effective is this new vaccine Cervavac and what obstacles lie ahead?
What is Cervical Cancer?
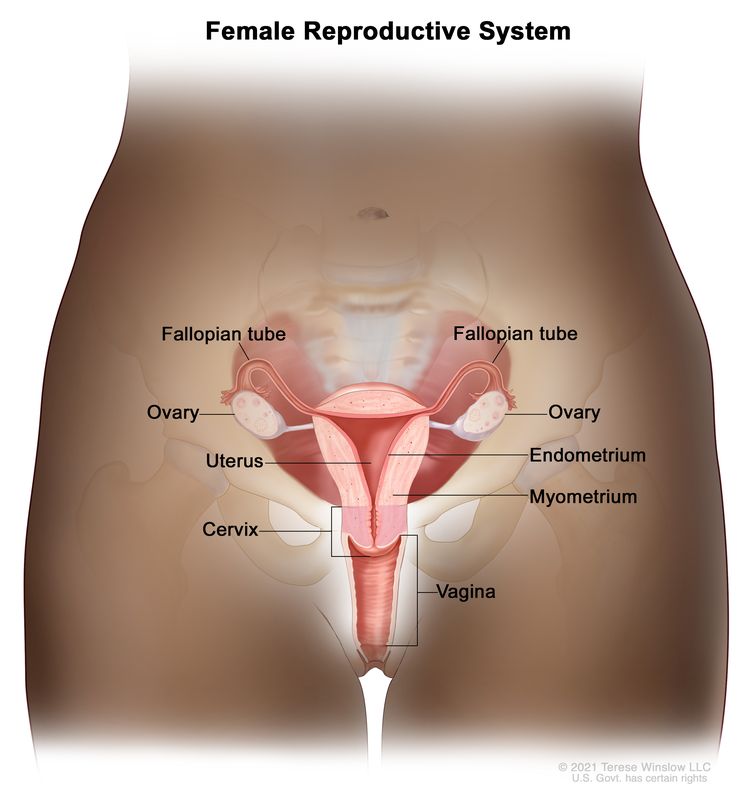
Cervical cancer is a type of cancer that starts in the cervix, the lower, narrow end of the uterus that connects the uterus to the vagina, and usually develops slowly over time.
Government Announcements
Cervical cancer, which is largely preventable, is the fourth most frequent malignancy in women worldwide with an estimated 604000 new cases and 342000 deaths in 2020.
On 1st September 2022, Union Minister Dr. Jitendra Singh announced the scientific completion of Cervavac, India’s first indigenously developed quadrivalent human papillomavirus (qHPV) vaccine for the prevention of cervical cancer.
Dr NK Arora, chairman of the Covid working group, National Technical Advisory Group on Immunisation said that although there are already two or three businesses producing the Cervavac vaccine in India, regulatory approval has already been received by Serum Institute of India (SII).
The prices of this Cervavac vaccine are still unknown, but it is estimated that the vaccine’s price will be one-tenth that of the best-selling, internationally recognized vaccination.
Doses and effectiveness of the vaccine
Government may roll out HPV vaccination to girls between the ages of 9 and 14 as part of its national health program. Cervical cancer can be cured completely by vaccination.

According to Rajesh Gokhale, the antibodies that develop after both doses of vaccines can last up to six to seven years also unlike COVID booster shots may not be needed. Until recently, the HPV vaccinations sold in India were made by foreign companies that range between Rs 2,000 and Rs 3,500 for each dosage.
Cervavac is anticipated to cost between Rs 200 and 400, making it much cheaper.
Who developed the new qHPV vaccine?
The Bill and Melinda Gates Foundation collaborated with the partnership program ‘Grand Challenges India’ of DBT and BIRAC to create “CERVAVAC,” funded by the Serum Institute for the development of indigenous people.
A total of 250 representatives from South Asian nations are now participating in a meeting of 50 countries to discuss the cervical cancer and HPV prevention landscape.
In 2011 Dr. M K Bhan, who was the DBT’s then secretary, carried out the initiative to create the vaccine. Since then, 30 scholarly advisory group conferences and site visits by DBT have assisted in evaluating the scientific validity of the whole vaccine development process.
What are the challenges?
The most difficult issue will be allocating enough funds to vaccinate the vast population of adolescent girls between the ages of 9 and 15, in order to ensure that they are protected from HPV at an early age.
Expert Opinions
It is alarming that the population has such low levels of knowledge and screening about cervical cancer. A massive awareness campaign is needed since this is a preventable disease. Experts predict that school-based vaccination programs will be beneficial. India accounts for the majority of cervical cancer fatalities worldwide with 80,000 new cases per year.
However, it will be necessary to make a structural approach to guarantee that NGOs and private healthcare institutions are included for the rollout to be successful.













