The World Health Organization stated on Wednesday that the deaths of scores of young children in Gambia from acute kidney damage may have been caused by tainted cough syrup and cold syrups manufactured by a medicine company based in India.
The results were published by WHO Director-General Tedros Adhanom Ghebreyesus after tests on numerous medical syrups suspected of causing 66 infant fatalities in the tiny West African country.
The deaths of 66 children in the West African country of Gambia have put a pall over India’s $42 billion pharmaceutical sector, which Prime Minister Narendra Modi has pushed as the “pharmacy of the world.”
Tedros told reporters that the United Nations was undertaking an inquiry with Indian officials and the business that manufactured the syrups, Maiden Pharmaceuticals Ltd. of New Delhi. Maiden Pharma declined to comment, while calls and mails to India’s Drugs Controller General remained unanswered. The Indian Ministry of Health did not reply to a request for comment.
On Wednesday, the WHO issued a medical product warning requesting that Maiden Pharma products be removed from the market.
The goods may have been spread worldwide through informal marketplaces, but the WHO claimed in its advisory that they had only been found in Gambia.
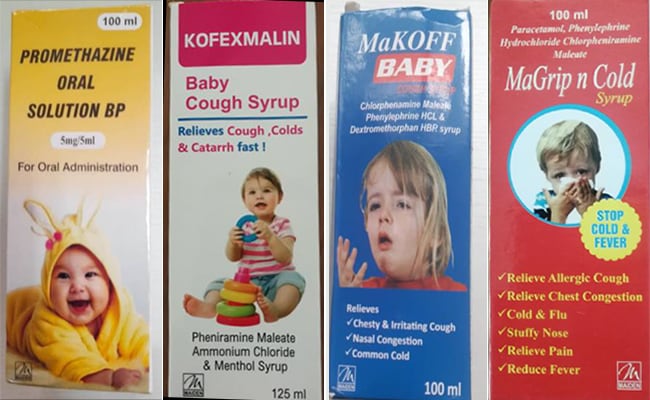
Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup are the four items covered by the warning.
According to the WHO, lab examination revealed “unacceptable” levels of diethylene glycol and ethylene glycol, which can be poisonous and cause acute kidney impairment.
In July, medical experts in Gambia issued an alert after hundreds of toddlers were ill with renal issues. The fatalities perplexed doctors until a pattern emerged: scores of youngsters under the age of five were unwell three to five days after swallowing a locally marketed paracetamol syrup.
Mustapha Bittaye, the Gambia’s head of health services, stated that similar concerns have been found in other syrups but that the ministry is awaiting confirmation of the results.
He stated that the number of deaths has decreased in recent weeks and that the sale of Maiden Pharmaceuticals goods has been prohibited. However, he claims that some of the syrups were still being offered at private clinics and hospitals until recently.
On Tuesday, the Gambia’s Medicines Control Agency issued a letter to health professionals instructing them to stop distributing any of the WHO-listed medications.
Probe Launched
Haryana’s government has submitted samples of four cough syrups made by a Sonipat-based company to the Central Drugs Laboratory in Kolkata for testing, state Health Minister Anil Vij announced on Thursday, October 6, 2022.
“The samples were gathered by a team from the Drugs Controller General of India (DCGA) and Haryana’s Food and Drugs Administration Department and transported to the CDL in Kolkata for analysis,” Vij explained.
“The cough syrups prepared by the pharma business were allowed for export,” Vij added. It is not for sale or distribution in the nation. Whatever action is required will be performed after the CDL report is received. Only until the report is completed will we be able to determine if the deaths in The Gambia were caused by these drugs or by some other factor.”
When asked about it at a press conference, Chief Minister Manohar Lal Khattar stated the Centre was looking into it.
Cough Syrup Company is a Habitual Offender
According to their website, Maiden Pharmaceuticals makes pharmaceuticals in India and distributes them both locally and to nations in Asia, Africa, and Latin America.
Many of the low-cost, generic medications supplied in American pharmacies and in hundreds of other nations come from India. However, South Asian remedies have been the basis of several manufacturing controversies in recent years, including the export of faulty cardiac medications.
The WHO has cautioned that four “contaminated” and “substandard” cough syrups purportedly made by Maiden Pharmaceuticals Limited in Haryana’s Sonipat might be the cause of the fatalities in West Africa.
According to its website, Maiden Pharmaceuticals has two production units in Kundli and Panipat, both in Haryana state near New Delhi, and has just opened a third. According to its website, company has been manufacturing and delivering medical items for more than 30 years and has a presence in Africa, Asia, Eastern Europe, the Middle East, and Russia.
It can produce 2.2 million syrup bottles, 600 million capsules, 18 million injections, 300,000 ointment tubes, and 1.2 billion tablets each year.
It has been found that Maiden has repeatedly produced substandard drugs in the past, yet was allowed to continue to operate. According to the eXtended Licensing, Laboratory and Legal Node (XLN) database maintained by the Government of India, at least two state governments – Kerala and Gujarat – have repeatedly warned of the company’s illegal practices.
Kerala authorities found Maiden’s products to be of substandard quality at least five times in 2021 and 2022. All of these units had been manufactured in Haryana.
Health department officials had picked up tablets of metformin – used to treat type-2 diabetes – from a taluk headquarter hospital in Palakkad in March 2022 and from a primary health centre in Ernakulam in September 2022. Both sets failed the dissolution test: the drug wasn’t able to dissolve properly in a given amount of time, thus failing to release the active ingredient into the body.