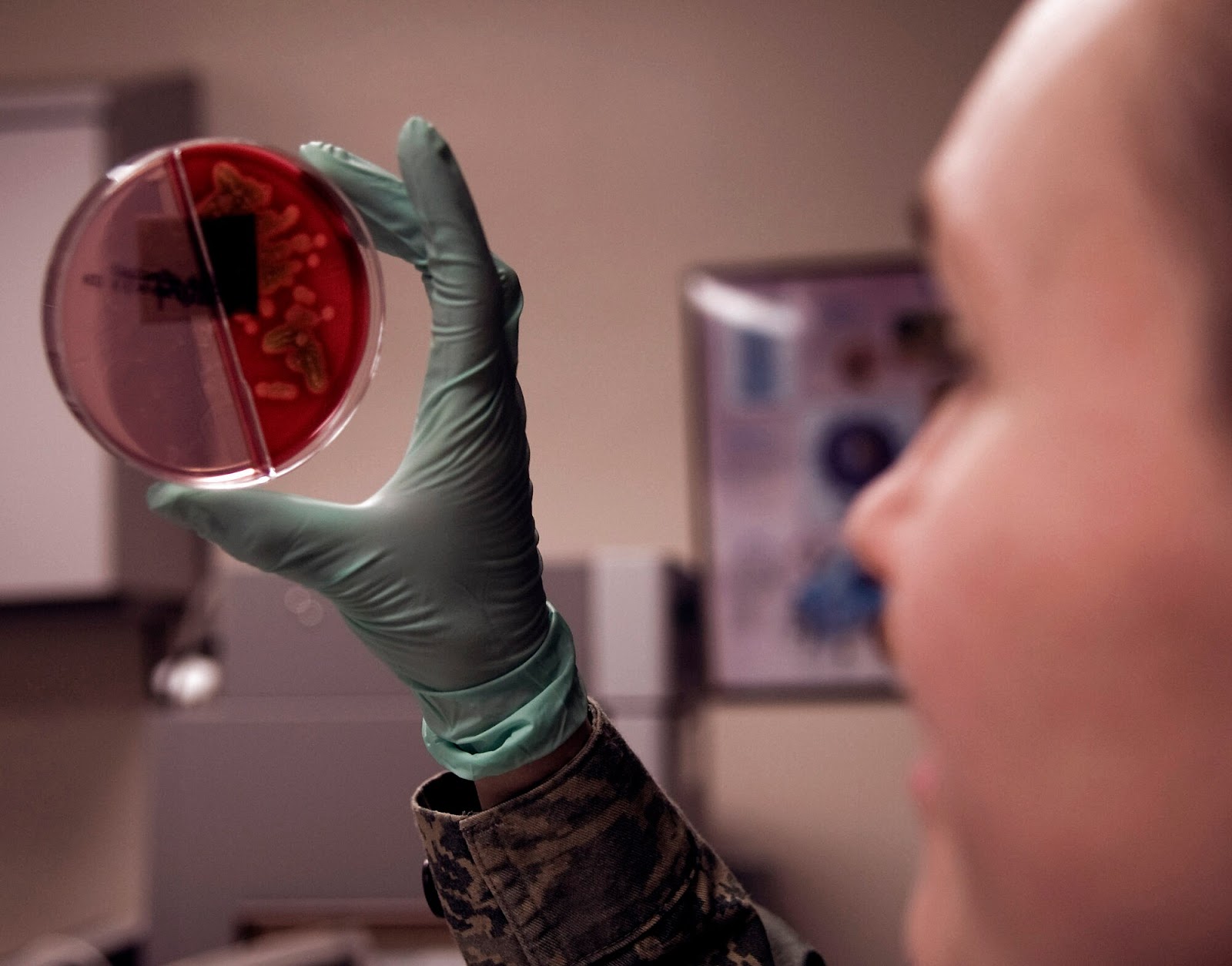
Antibiotic-resistant bacteria have been alarming, leading scientists to pursue more resilient and alternative strategies and molecules.

The rise of antibiotic-resistant bacteria is a global concern, prompting scientists to explore alternative strategies. In response, researchers at the Indian Institute of Science (IISc) have achieved a significant breakthrough by designing a peptide capable of neutralizing key enzymes in the deadliest and most antibiotic-resistant bacteria.
Antimicrobial resistance poses one of the most significant threats worldwide, causing an alarming 4.95 million deaths in 2019. The culprits behind these fatalities are bacteria like Staphylococcus aureus, Streptococcus pyogenes, Pseudomonas aeruginosa, and Escherichia coli, which have developed resistance to antibiotics.
The newly developed peptide, consisting of just 24 amino acids, can effectively inhibit a class of enzymes known as Topoisomerases. These enzymes are vital for bacterial replication and protein synthesis. By targeting them, the growth of dangerous bacteria is halted, preventing further proliferation.
The attractiveness of these enzymes as target sites lies in their difference from those found in humans. Current antibiotics like ciprofloxacin target topoisomerases, forming covalent adducts, which are intermediate complexes in bacterial DNA for coiling and uncoiling. The peptide molecule created by IISc researchers binds to these adducts, trapping them and triggering a series of events that ultimately lead to bacterial cell death. Professor Raghavan Varadarajan from the Molecular Biophysics Unit (MBU) explains the mechanism.
The designed peptide shares similarities with a natural toxin called ‘CcdB,’ produced by certain bacteria and plasmids. However, the CcdB protein is too large to be an effective drug molecule. To address this, the research team modified the peptide by snipping a small section from the CcdB protein’s tail end and adding a few amino acids, enabling the peptide to enter bacterial cells. The new peptide’s efficacy was tested on disease-causing bacterial species such as E. coli, Salmonella Typhi, Staphylococcus aureus, and a multi-drug resistant strain of Acinetobacter baumanii. Both cell cultures and animal models were used for the tests, in collaboration with Professor Dipshikha Chakravortty’s lab from the Department of Microbiology and Cell Biology (MCB). The effects of the new peptides were also examined in combination with ciprofloxacin.
The results demonstrated that the peptide successfully inhibited or poisoned the topoisomerases, while also disrupting the bacterial membrane. Most notably, the growth rate of bacterial load declined significantly. For example, in animals infected with antibiotic-resistant Acinetobacter baumannii, the peptide treatment led to an 18-fold reduction in bacterial load in the liver, compared to only a 3-fold reduction achieved by ciprofloxacin. Importantly, the new peptide exhibited no toxic effects and was deemed safe for use in animal models.
This groundbreaking research opens up new possibilities in the fight against antibiotic-resistant bacteria by targeting topoisomerase enzymes. The researchers believe that this peptide could be used in combination with existing antibiotics, presenting a promising avenue to combat antimicrobial resistance effectively.
In conclusion, the researchers at IISc have designed a synthetic peptide that shows great promise in tackling antibiotic-resistant bacteria. This innovation represents a significant step forward in addressing the global health challenge posed by antimicrobial resistance. By targeting key enzymes in these deadly bacteria, this peptide offers a potential solution to save millions of lives and preserve the effectiveness of current antibiotics. The potential use of this peptide as a combined therapy with existing antibiotics presents a promising approach for the future of antimicrobial treatments.