Researchers from the Baker Heart and Diabetes Institute in Australia have reported a breakthrough achievement in type 1 diabetes research. Combining GSK-126, a common anticancer drug, with triptolide, a naturally derived compound with anti-inflammatory effects, they were able to stimulate pancreatic ductal cells to evolve into insulin-producing cells. The insights obtained from this study can lead to the development of an alternative method of reviving insulin production in individuals suffering from type 1 diabetes.
The Global Diabetes Pandemic
Globally, 8.75 million people were surviving with type 1 diabetes in 2022, as reported by the International Diabetes Federation. In this disease, the immune system of an individual damages or kills the beta cells of her pancreas, thus hampering insulin production. The individual ends up with inadequate or no insulin production.
Once we have a meal, the carbohydrates in the food are broken down into glucose, a sugar that acts as the primary source of our energy. The produced glucose enters the bloodstream and gets circulated. It is at this point that the pancreas becomes active by releasing the insulin hormone. This crucial hormone can be imagined as a ‘key’ that opens up the ‘doors’ of the body cells so that the glucose molecules can enter. Inside the cells, glucose is converted to energy that is consumed to run the body’s machinery.
In type-I diabetes, glucose cannot enter the cells, and the blood glucose level continues to rise after eating. Naturally, the first line of treatment would be the regeneration of the beta cells so that they produce insulin again. For decades, researchers have been catering efforts to achieve this; in fact, they attempted to convert stem cells from other parts of the body into insulin-producing cells.
Pancreatic Beta Cells: The Insulin Factories
As an extremely active gland inside our body, the pancreas performs two primary functions: exocrine, that is, the production and secretion of enzymes, and endocrine, that is, the generation and release of hormones, such as insulin.
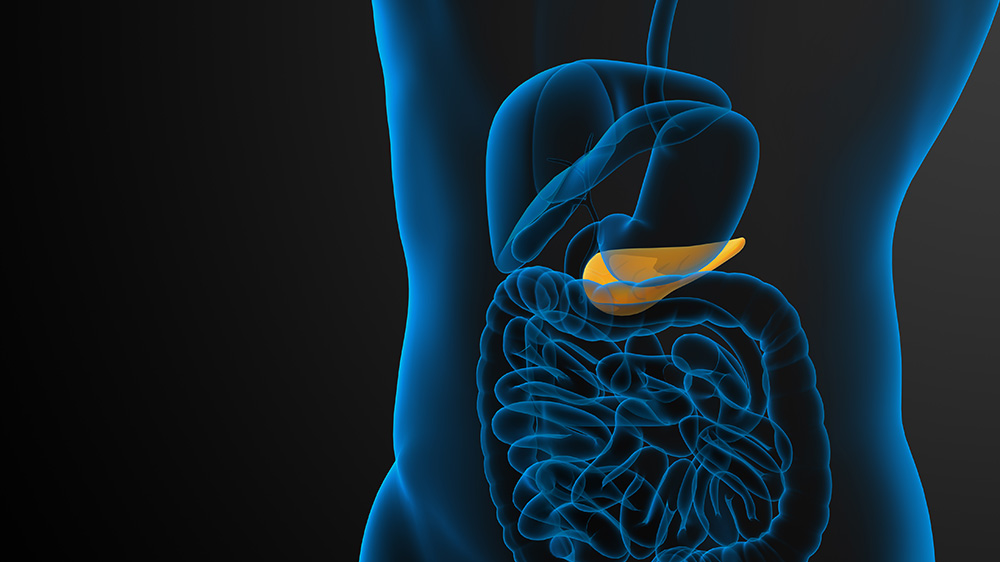
The exocrine cells that form the lining of the ducts that deliver pancreatic enzymes are known as the ductal cells. Several studies have reported that the ductal progenitor cells, which are the descendants of stem cells, bear the potential to differentiate into insulin-producing beta cells.
Cancer Drug Revives Dead Beta Cells
To realize this, the researchers at the Baker Heart and Diabetes Institute conducted the current study using the GSK-126–triptolide combination. Mechanistically, GSK-126 is an EZH2 enzyme inhibitor. The EZH2 gene gives instructions for the production of an enzyme named EZH2, which constitutes a part of a protein group, the polycomb repressive complex-2.
This complex turns off particular genes and thereby participates in the process of determining the fate of an immature cell. Thus, polycomb repressive complex-2 plays an instrumental role in determining what type of cell an immature cell will eventually mature into.
Results from previous studies are available that suggest the pancreatic duct cells are a source of progenitors for beta cell regeneration. With this cue, the researchers of this study inhibited the EZH2 enzyme in the pancreatic tissues obtained from donors using the GSK-126–triptolide combination.
Surprisingly, they observed that the ductal cells functionally resemble the beta cells and when exposed to glucose solution, they produce insulin. On measuring the expression of the insulin gene and genes related to conferring the progenitor identity, they found that all these genes were expressed in both diabetic and nondiabetic individuals.
Looking Further Towards a Treatment
“More work is required to define the properties of these cells and establish protocols to isolate and expand them,” said Al-Hasani, a member of the research team.
What makes the scientific community excited is the hope for the regeneration of the beta cells and the revival of their insulin production. However, further studies are necessary to gain an in-depth understanding of the drug mechanism and develop current insights into practical treatment options.