An intriguing discovery which involved rejuvenation of kidney cells was achieved by the scientists at the University of Texas. The study detailing this mechanism was published in Nature Nanotechnology. A previously unknown phenomenon of housekeeping, in which kidney cells expel out undesirable material, which helps existing cells to renew themselves and sustain longevity and health of kidneys throughout the lifetime is coming into light through this breakthrough research.
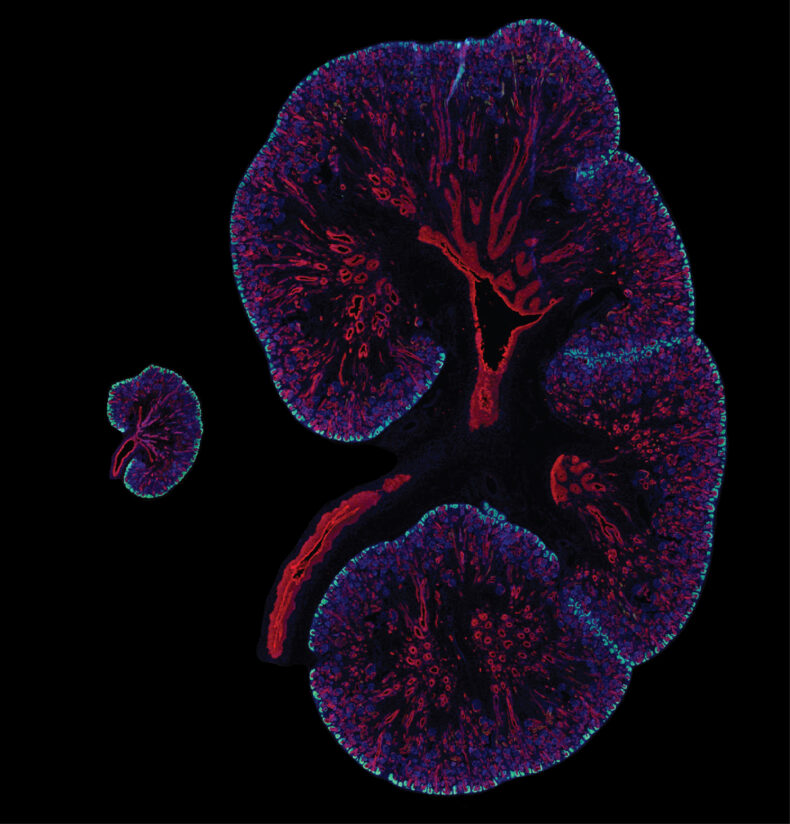
While the liver and skin generate new cells through cell division, the cells in the proximal tubules of the kidney remain quiescent and do not divide. However, in cases of mild injury or disease, limited repair capabilities exist, and kidney stem cells can contribute to the formation of new kidney cells to only some extent.
Dr. Jie Zheng, one of the lead researchers of the study further enlightens the mechanism. In typical cases, severe injury to kidney cells results in their death without the ability to regenerate. Ultimately, this leads to kidney failure, presenting a major challenge in the management of kidney diseases. Currently, the best approach in use is to slow down the progression towards kidney failure rather than readily repairing the organ when faced with severe or chronic damage.
This is precisely why the discovery of the self-renewal mechanism holds immense significance as it offers new possibilities.
A Rejuvenating Approach
The extrusion-based self-renewal mechanism represents a significant departure from known regenerative processes, such as cell division, as well as exocytosis, which involves the encapsulation and release of foreign substances. Unlike these processes, the discovered mechanism does not involve membrane fusion. Instead, it allows normal cells to eliminate old content and refresh themselves with new contents, regardless of the presence of foreign nanoparticles. This intrinsic and proactive process plays a crucial role in extending cell survival and maintaining proper function.
These findings present new avenues for exploration. Epithelial cells, like those in the proximal tubules, are found in various tissues, including artery walls, the gut, and the digestive tract. Understanding how nanoparticles are eliminated from the proximal tubules is particularly relevant in the field of nanomedicine, aiming to minimize their accumulation in the body. Additionally, by unraveling the regulation and monitoring of this self-renewal process, potential approaches for maintaining kidney health in patients with conditions like high blood pressure or diabetes could be identified.
Noninvasively detecting the signature of this process could serve as an early indicator of kidney disease, providing valuable insights for diagnosis and treatment.
Research that led to a Rejuvenating Discovery
For around 15 years Zheng and his team have studied the biomedical applications of gold nanoparticles, including their use as imaging agents and drug delivery systems. Their efforts centered on comprehending the underlying mechanism of filtration and elimination of nanoparticles through the kidneys.
Previous research has shown that gold nanoparticles pass through kidney’s glomerulus and are then taken up by the proximal tubular epithelial cells before being excreted out in urine.
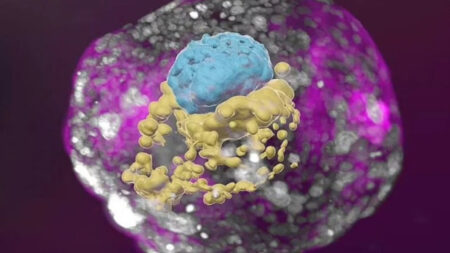
To better understand the concept, the researchers used an electron microscope in their recent study to investigate gold nanoparticles in proximal tubular tissue samples. They were taken up by surprise with the results. They found that the nanoparticles were encapsulated within lysosomes which were confined in large vesicles present in the extracellular space outside epithelial cells. The extruded contents were then pinched off and released into the extracellular space.
It was then, when the scientists understood the existence of such a novel mechanism which could represent a new way for cells to eliminate cellular contents and ensure longer vitality of healthier cells.