Researchers have recently developed and characterised two monoclonal antibodies (NA8 and NE12) that have neutralising efficacy against the main SARS-CoV-2 variants of concern and target the receptor-binding region of the severe acute syndrome coronavirus type 2 (SARS-CoV-2) spike protein.
The emergence of new treatment methods has resulted in a dramatic change in the drugging of previously undruggable targets. Molecular and RNAi therapies, recombinant proteins, viral gene therapies, antisense oligonucleotides, cellular immunotherapies, ex vivo gene therapies like CAR-T cells, the microbiome, PROTACs, and other novel therapeutic modalities are all part of a revolution that started with the therapeutic use of monoclonal antibodies in the 1970s. Patients suffering from conditions like cancer, Alzheimer’s disease, multiple sclerosis, and other conditions that were considered incurable are finding fresh hope because of these cutting-edge treatment options. According to research, complicated tasks can be completed by innovative treatment modalities in ways that conventional medications cannot.
Although the coronavirus disease 2019 (COVID-19) pandemic’s morbidity and mortality have been significantly reduced by the quick development of vaccines and monoclonal antibody treatments, the emergence and widespread dominance of SARS-CoV-2 Omicron subvariants carrying mutations that facilitate immune evasion is a cause for growing concern.
There are 30 mutations in the SARS-CoV-2 Omicron (BA.1) variation, 15 of which are in the receptor binding region, the area that is most frequently targeted by neutralising antibodies. In terms of worldwide prevalence, subvariants BA.4 and BA.5 have supplanted the other variations. They also show resistance to neutralising antibodies produced by vaccinations and earlier SARS-CoV-2 infections.
In order to combat the emerging Omicron subvariants, new potent antibody treatments are required because available clinical monoclonal antibodies have diminished efficacy against these subvariants.
Regarding the study
To create the phage-display fragment antigen-binding (Fab) libraries in the current work, the researchers employed ribonucleic acid (RNA) taken from the PBMC (peripheral blood mononuclear cells) of 12 COVID-19 patients who had a high antibody titer of neutralising antibodies against SARS-CoV-2.
The trimeric spike protein was particularly targeted by enzyme-linked immunosorbent assays (ELISA), which were used to find clones that bind to it. Clones with distinctive sequences were found using deoxyribonucleic acid (DNA) sequencing. These clones were subsequently produced as soluble Fabs and full-length immunoglobulin G1 (IgG1) antibodies. Surface plasmon resonance was utilised to assess the binding ability of the Fabs towards the trimeric spike protein, whereas ELISA was employed to examine the binding affinity of the IgG antibodies.
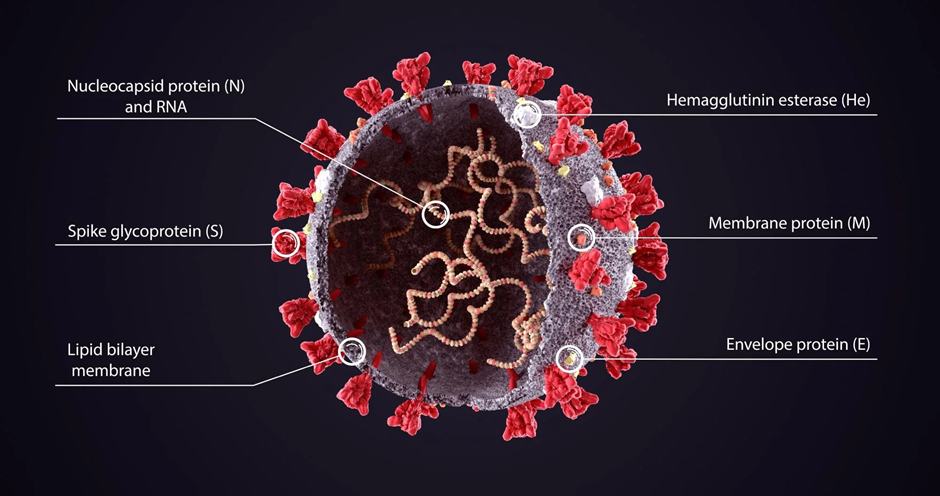
Evaluations of the chosen monoclonal antibodies were carried out using pseudotyped viruses in neutralisation experiments. Using competitive ELISA, the monoclonal antibodies’ specificity for the receptor binding region of the spike protein was evaluated. Additionally, the North American founder strain, WA-1, and other pertinent variations of concern were examined for their capacity to neutralise monoclonal antibodies. Additionally, the neutralising power of the chosen monoclonal antibodies was contrasted with that of monoclonal antibodies currently being used in clinical settings.
The structural interactions between the two most powerful monoclonal antibodies from the study—NA8 and NE12—and the SARS-CoV-2 spike protein trimer were studied using cryogenic electron microscopy (cryo-EM).
The in vivo preventive effectiveness and therapeutic activities of NA8 and NE12 were also examined using hamster models. Before being intranasally challenged with the beta variation and WA-1 strain of SARS-CoV-2, the hamsters were intraperitoneally infected with various quantities of monoclonal antibodies to determine the preventive effectiveness. Following infection with the WA-1 strain and beta variation, the hamsters were intraperitoneally given monoclonal antibodies to assess the effectiveness of the treatment.
Results of the experiment
According to the findings, NA8 and NE12 demonstrate complimentary traits and powerful neutralising activity against all of the key SARS-CoV-2 variants of concern. At picomolar concentrations against the Beta variation and the Omicron BA.1 and BA.2 subvariants, as well as at nanomolar concentrations against the more modern BA.2.12.1 and BA.4 subvariants, the NA8 monoclonal antibody demonstrated wide neutralising powers.
According to the cryo-EM structural analysis pictures, the NA8 antibody may avoid the altered portions of the Beta and Omicron receptor binding regions thanks to a highly conserved binding epitope and a slightly displaced receptor binding ridge. The angiotensin-converting enzyme-2 (ACE-2) receptor footprints of the receptor binding domain were observed to coincide with the NE12 epitope. The combined usage of NA8 and NE12 did not show any loss in the neutralising power of either of the monoclonal antibodies, despite the largely overlapping epitopes.
When evaluated in hamster models, the two monoclonal antibodies also showed great preventive and therapeutic efficacy in vivo. Furthermore, NA8 and NE12 have shown a robust and widespread neutralising effect in comparison to the monoclonal antibodies currently being used in clinical settings.
Conclusion of the Study
To sum up, the study described the creation and characterization of two new monoclonal antibodies, NA8 and NE12, which, when combined, demonstrated wide and powerful neutralising powers against the main SARS-CoV-2 variants and the dominant Omicron subvariants.
Additionally, the two monoclonal antibodies demonstrated strong therapeutic and preventative effects in vivo in hamster models. The extensive potency and reactivity were explained by the structural study of the binding epitopes. The conserved binding epitope of NA8 also suggests that it has the potential to deactivate new variations.
The creation of widely neutralising antibodies like NA8 and NE12 will help stop the global spread of immune-evading SARS-CoV-2 variations because currently employed monoclonal antibody treatments and vaccines are no longer effective against the emerging Omicron subvariants.
Read more on: https://tdznkwjt9mxt6p1p8657.cleaver.live/covid-is-still-an-international-emergency-says-who/